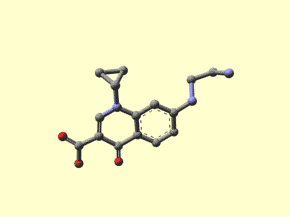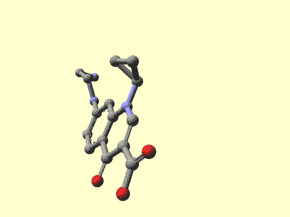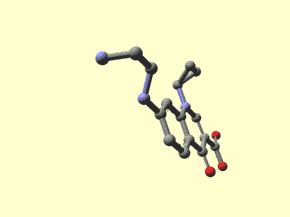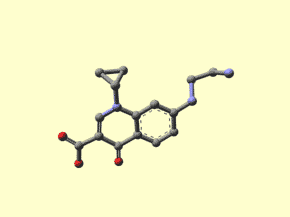
Dan's Chemistry Page
The Beauty of Molecules
Molecules have been divided into 2 main classes: Inorganic and Organic. The main difference between these two classes is that "organic" molecules contain a skeleton made of carbon atoms as is found in living matter. This would lead one to believe that all organic molecules come from nature. In fact, chemists have known how to synthesize organic molecules for about a century. But whether a molecule comes from nature or a chemist's lab, if it contains a skeleton of carbon atoms it is classified as organic.
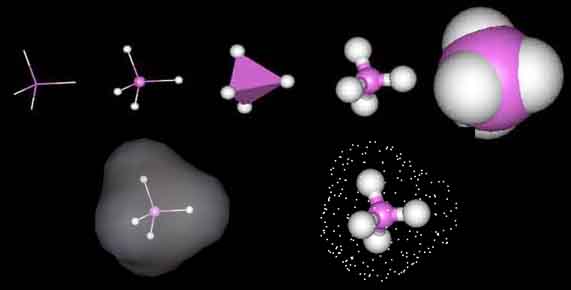
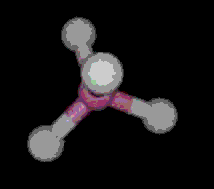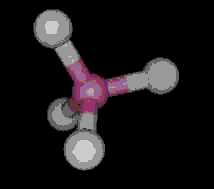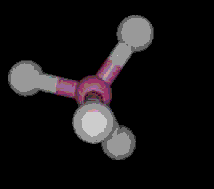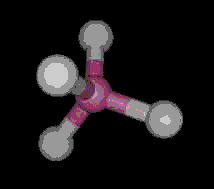
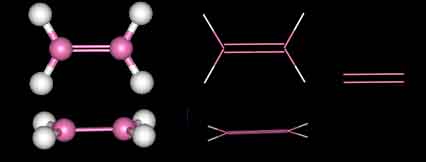
The most simple organic molecule is methane, a flammable gas which occurs naturally during the decomposition of organic matter. The skeleton of methane is composed of a single carbon atom. Most carbon atoms have four bonds symmetrically placed around them which link them to other atoms. In the case of methane, the carbon atom is linked to four hydrogen atoms forming a perfect tetrahedral (four-sided) pyramid. The above images show various ways of representing methane with and without its electron cloud. These images were all drawn by artists as molecules are too small to be photographed. In fact, molecules are too small to be seen with the most advanced microscopes. Experiments are performed to determine the positioning of the atoms from which their structures and shapes can be deduced.
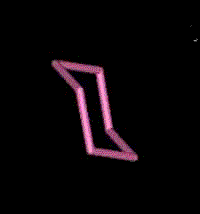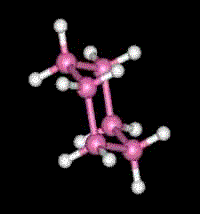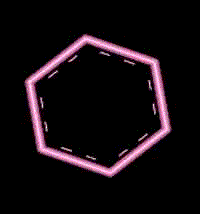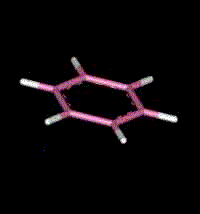
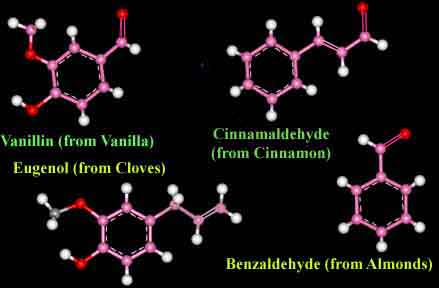
A molecule formed with two carbon atoms has usually six bonds available to bond to other atoms. In the case of ethylene, a flammable gas used to make polyethylene, there are two carbon atoms linked together by two bonds callaed "double bond". In the geometry of the ethylene molecule, the two carbon atoms form a planar (flat) structure rather than the tetrahedral structure formed by the methane molecule. Cyclo-Hexane consists of six carbon atoms linked together by single bonds to form a ring. This structure cannot be flat since each of the six carbon atoms bonds to two other carbon atoms forming a ring of six tetrahedral structures. Benzene is formed by a similar structure of six carbon atoms in a ring, except that they are linked by double bonds like those used in the planar structure of ethylene. Thus the entire structure remains flat. Benzene is the most simple aromatic molecule, named so because it was long ago found to be part of the structure of the aroma of vanilla, cinnamon, almond, clove, and many other aromatic flavors. Actually, millions of molecules which are formed by this ring structure continue to be called "aromatic" even though they have no smell at all. This mis-labeling is common to many old names of chemistry compounds. (Code: red atoms = oygens, pink = carbons, white = hydrogen)
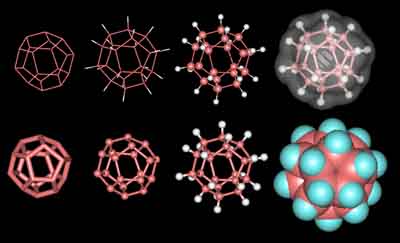
Dodecahedrane
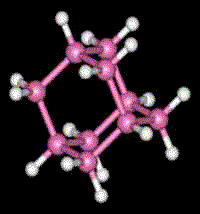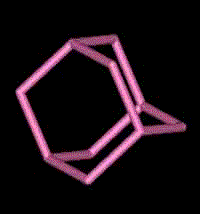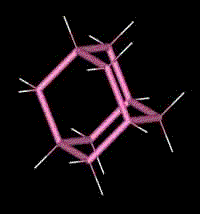
Adamantane
Below are two synthetic molecules which are particularly interesting because of their symmetrical structures:
1) Dodecahedrane is composed of 12 fused cyclo-pentane rings. It is represented by eight still images, some of which show the hydrogen atoms.
2) Adamantane is composed of four fused cyclo-hexane rings. As you can see in the animated representation, it has similarities with the structure of diamond.
Two very different natural products.
The spinning structure is Luciferin. This small molecule uses oxidation to produce the light in fireflies. You can see that part of the molecule is flat. This is the so called "aromatic" area which contains rings with double bonds, including a benzene ring.
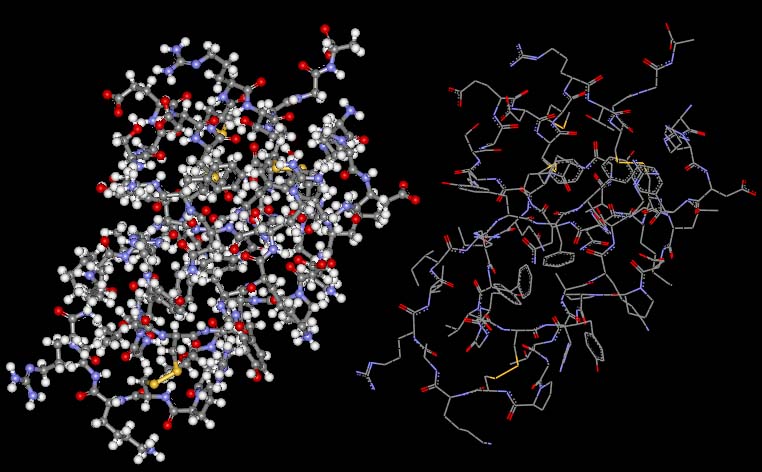
The second molecule, Trypsin, is a very large and complex enzyme that is produced in the small intestine. It accelerates the cleavage of proteins into amino acids which can then be reabsorbed through the intestine. The image on the left is complete with the hydrogen atoms found in Trypsin. In the image on the right, the hydrogen atoms have been left out to create a better view of the molecule's three-dimensional structure.
Code: grey atoms = carbons, white = hydrogens, red = oxygens, blue = nitrogens and yellow = sulfurs.
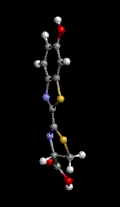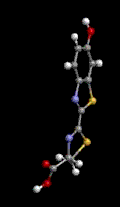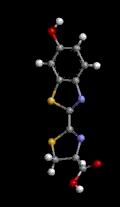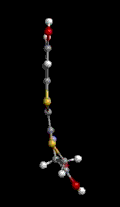
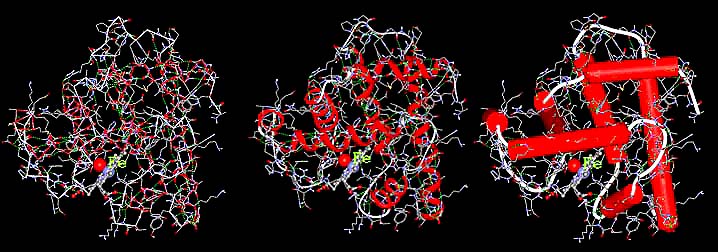
Hemoglobin is the red blood cell protein which picks up oxygen (O2) from air in the lungs, stores it, and then distributes it throughout the body where it is needed. Iron plays a very important role in maintaining the oxygen within the giant hemoglobin molecule and releasing it in the proper places. In the three representations of hemoglobin, below, oxygen is represented by the red ball and iron is represented by the violet ball. The official chemical symbol for iron is Fe, from the French Fer.
Oxygen is used, for one example, to "burn" sugars in the muscles producing both energy and carbon dioxide (CO2). The same hemoglobin molecules then take the carbon dioxide back to the lungs to be exhaled from the body. Some toxic gases can block this process. For example, carbon monoxide (CO), formed by the incomplete combustion of gasoline or any other carbonaceous material, holds so strongly to the iron-hemoglobin complex that it is inhibited from carrying oxygen.
Proteins consist of very long chains of atoms. Their primary structure provides only the linkage between the atoms. They also have a secondary structure which causes them to be rolled like a rope or a screw. This secondary structure is flexible and if distorted will return to its original shape. Though these "screw" patterns are drawn in red, they are still difficult to see in the illustration on the left. In the middle illustration they have been drawn with wide red lines to make them more visible. And in the illustration on the right, each screw pattern has been replaced by a red tube of the same length.